Abstract
Chronic rhinosinusitis (CRS) is a common health care problem, yet many aspects of this diagnosis remain poorly understood. Its etiology is often debated and remains a significant area of research. The diagnosis of CRS is based on subjective symptoms, duration of symptoms and objective evidence of inflammation. Each of these criteria must be met to make a diagnosis of CRS. Management of CRS often involves a combination of systemic and topical therapies with surgery reserved for patients who fail medical therapy. This review provides a comprehensive view of the etiology, diagnosis and management of CRS.
Introduction
Chronic rhinosinusitis (CRS) represents one of the most common healthcare problems in the USA, afflicting approximately 31 million Americans.[1] CRS is a clinical syndrome associated with persistent inflammation of the mucosa of the nose and paranasal sinuses for 12 weeks or longer.[2,3] It is known to cause significant physical impairment, adversely impacting patient quality of life and psychosocial well-being. Despite its prevalence, CRS remains a challenging and, at times, controversial, disease entity. The etiologic mechanisms of CRS continue to be a source of much debate and, as such, different schools of thought exist on the optimal management strategy. The purpose of this review is to describe the different proposed pathophysiologic mechanisms of CRS as well as review the diagnostic and treatment strategies for the management of this complex disease.
Accurate diagnosis of CRS rests on the ability to identify signs and symptoms associated with the disease process, such as nasal obstruction, purulent discharge and/or facial pain, as well as objective evidence of mucosal inflammation, either by nasal endoscopy and/or computerized tomography.[4]However, it is also important to recognize that this is a heterogeneous disease spectrum subject to further subclassifications. Patients with CRS may be divided between CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). This distinction leads to both clinical and pathological differences. CRSwNP is predominantly mediated by eosinophils, as well as increased levels of histamine, IL-5 and IL-13.[5] By contrast, CRSsNP seems, at first glance, to be predominantly mediated by neutrophilic inflammation.[6] However, some CRSsNP cases may also exhibit extensive eosinophilic infiltration. Therefore, the distinction between CRS with and without polyps is not as clear as originally thought. In addition, CRS must be clearly differentiated from systemic processes that lead to sinonasal mucosal inflammation. Clinical entities, such as cystic fibrosis, sarcoidosis, Wegener's granulomatosis and primary immunodeficiency (PID) may present with sinus involvement as a component of the multisystem process. Some cases of PID can be relatively mild and manifest primarily as sinusitis without pneumonia or other more serious systemic infections. The prevalence of PID in patients with recalcitrant CRS varies widely in the literature, from 0 to 19%. Furthermore, secondary CRS may arise as a result of local, discrete processes such as tumor, mycetoma and foreign body reaction. A recent study even suggests a potential causal relationship between tobacco smoke exposure and the development of CRS.[7] The primary focus of this review is to discuss CRS as a primary disease process in the absence of systemic or local predisposing factors.
Etiologies of CRS
While the main underlying cause of acute rhinosinusitis (ARS) is probably bacterial in origin, the central pathophysiologic mechanisms of CRS remain to be fully elucidated. Several possible mechanisms have been proposed, including microbes (bacteria, fungi, viruses), biofilm formation, staphylococcal superantigen (most often associated with CRSwNP), osteitis and derangements in innate and adaptive immunity.
Viruses
Patients with CRS commonly report that their symptoms initially started after an acute viral event.[8] Furthermore, viruses can cause multiple changes on a cellular level, consistent with etiologies of CRS, such as increase in bacterial adhesion and production of inflammatory mediators by nasal epithelial cells.[9,10] Multiple studies have evaluated the presence of respiratory viruses in samples taken from patients with CRS. Ramadan et al. found respiratory syncytial virus (RSV) present in 20% of samples collected from patients with CRS.[11] However, this study did not report a control group, nor the timing of the specimen collection, as the presence of RSV is much greater in the winter months in the general population. Jang et al. reported a similar study with a control group and collected specimens during the summer months.[12] Rhinovirus was identified in 21% of samples from CRS patients and 0% in the control group. However, these samples were taken from the inferior turbinates and not the paranasal sinus mucosa. By contrast to the above studies, Wood et al. collected sinus mucosa samples from 13 CRS patients and two controls.[8] No respiratory viruses, including RSV and rhinovirus, were identified in any of the samples. While viruses may be implicated in the initial or ongoing stimulus of inflammation, their exact role in CRS is not clearly defined.
Bacteria
The most common bacteria identified in ARS are Streptococcus pneumonia, Moraxella catarrhalis andHemophilus influenza.[13] This is in direct contrast to the predominant organisms identified in CRS. Kingdom and Swain evaluated 182 total cultures in 101 patients at the time of sinus surgery.[14] The most common organisms identified were coagulase-negative Staphylococcus (SCN) (45%), Gram-negative rods (25%) and Staphylococcus aureus (24%). Nadel et al. identified similar bacteria in 507 endoscopically guided cultures.[15] The predominant organisms included S. aureus (31.3%), SCN (44.2%) and Gram-negative rods (34.3%), including Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Escherichia coli and Serratia marcescens. These bacteria may be found in isolation but are often polymicrobial with two or more coexistent bacterial species.
Staphylococcus aureus & biofilms
S. aureus is present in patients with and without CRS, occupying the nasal vestibule of nearly a third of the human population at any given time. In addition to being frequently identified in patients with CRS, it is also associated with many community and hospital-acquired infections, including sepsis and endocarditis. While its presence is often noted in cultures of patients with CRS, its exact role in the etiology of CRS is unclear. S. aureus is often found in biofilms identified in patients with CRS. Singhal et al. collected sinonasal tissue samples in 39 patients undergoing functional endoscopic sinus surgery (FESS) for CRS.[16] Biofilms were identified in 30 out of 39 patients, and 70% of these biofilms had S. aureus present. Furthermore, patients with S. aureus biofilms had poorer symptom scores and quality-of-life outcomes and exhibited significantly worse nasal endoscopy scores postsurgery, when compared with those without S. aureus biofilms.
A biofilm is an organized community of bacteria adherent to an inert or living surface, embedded in a self-produced extracellular polymeric matrix composed of a mixture of biopolymers, primarily polysaccharides, but also containing protein and nucleic acid.[17,18] The presence of biofilms in CRS may have significant implications for treatment, as bacteria in the form of a biofilm may be resistant to antibiotic therapy. In addition to acting as a physical barrier preventing antibiotic penetration, biofilms may limit the effectiveness of antibiotics in other ways. The accumulation of waste products or oxygen in a biofilm can lead bacteria to enter a low metabolic state where they are much less susceptible to growth-dependent antimicrobial activity.[19] The use of efflux pumps and the downregulation of transmembrane channels or antimicrobial target sites may also lead to the ineffectiveness of antimicrobial therapy.[20,21] The sharing of genetic information and expression of antimicrobial-resistant genes may also lead to poor outcomes with the administration of antibiotics.[22] While biofilms are frequently seen in CRS, their presence alone does not implicate a potential causative role. However, recent studies suggest an interaction between biofilms and the adaptive immunity of the host, independent of the staphylococcal superantigen pathway. To date, this interaction remains undescribed, and further studies are required to determine the exact role of biofilms in the pathogenesis of CRS.
Staphylococcus superantigens
The superantigen hypothesis proposes that S. aureus secretes high-molecular-weight proteins known as enterotoxins. These enterotoxins have significant stimulatory activity that can foster the characteristic tissue response seen in patients with polyps. Approximately 50% of CRSwNP patients show lymphocyte responses consistent with superantigen exposure.[23] In addition, staphylococcal toxin-specific IgE antibodies have been detected in 18 out of 23 patients with nasal polyps.[24] It is unclear, however, whether S. aureus superantigens represent an etiologic agent or a disease modifier. The link between superantigens and CRSsNP has not yet been established.
Coagulase-negative Staphylococcus
The exact role of SCN in CRS is unclear, as its incidence varies widely. Bolger identified SCN in 17% of patients with CRS, while Hsu et al. found SCN in 42%.[25,26] However, these numbers must be weighed against other studies that identified SCN in the middle meatus of 56% of patients without CRS, and in only 20% of patients with CRS.[27] Furthermore, SCN is almost always present on human skin, leading to possible contamination without proper sterile technique during culture acquisition. Recent studies may point toward the need of a specific strain of SCN for an infection to develop. Bacterial pathogenicity may depend on genes associated with biofilm formation, only found in certain strains of SCN.[28]
Pseudomonas aeruginosa
Gram-negative rods are frequently identified in patients having undergone previous surgery. P. aeruginosa is a problematic organism, long recognized as an important pathogen in patients with cystic fibrosis. It is also identified in patients with CRS, with rates of assay reported between 9 and 16%.[14,15]One of the challenges of P. aeruginosa is that it also has the capability to form biofilms, which may in part lead to its refractory nature in patients with CRS. With limited orally administered antibiotics effective against P. aeruginosa, it represents an important treatment challenge in infectious exacerbations of CRS.
Stenotrophomonas maltophilia
S. maltophilia is a multidrug resistant Gram-negative bacillus often encountered in immunosuppressed and intensive care unit patients. It has been cultured from the paranasal sinuses, often in the setting of previous endoscopic sinus surgery and antimicrobial therapy. It is unclear whether this represents true infection or colonization after eradication of other bacteria by antimicrobial therapy. However, given its multidrug resistant nature, it should be considered in patients who have failed previous therapy.
Fungus
It is clear that fungus is responsible for some forms of sinusitis, in both invasive and noninvasive forms. Although a wide variety of fungi have been identified in the sinuses of CRS patients, the central etiologic role of fungus in CRS has not been clearly demonstrated. In 1999, positive fungal cultures from nasal mucus were used as the basis to posit that eosinophilic infiltration and fungal presence provided the main inciting event for CRS.[29] However, further studies found a similar percentage of positive cultures in normal control patients.[30] In addition, a double-blind, placebo-controlled randomized multicenter trial has failed to identify any benefit of topical antifungal therapy in objective and subjective outcome measures in patients with CRS.[31] A subset of CRS, allergic fungal rhinosinusitis (AFRS), is characterized by type I hypersensitivity to fungi, nasal polyposis, eosinophilic mucin, hyperdensities on computed tomography (CT) imaging and positive fungal stain or culture with the absence of diabetes, immunodeficiency or an invasive fungal process.[32] Furthermore, patients with AFRS have been shown to have elevated levels of total serum IgE and IgG anti-Alternaria antibodies when compared with patients with CRS.[33] While fungus does play a role in specific types of CRS, such as AFRS, its classification of the central pathophysiologic mechanism of CRS is not corroborated in the literature.
Osteitis
Osteitis is another possible etiologic factor for CRS. Patients with CRS often show areas of irregular bony thickening on CT imaging. It has been proposed that this irregular thickening and increased bone density may be a sign of inflammation of the bone, leading to persistence of inflammation of the overlying mucosa.[34] Osteitis is marked by varying degrees of osteoclast–osteoblast activity, leading to disruption of organized lamellar bone and leading to formation of immature woven bone.[35] Entry of inflammatory infiltrate into the increased haversian canal system may act as a potential pathway for spread of inflammation and, as such, mucosal disease. The prevalence of osteitis is estimated to be between 36 and 53% in CRS patients, based on CT findings or pathologic evaluation.[36] This concept of osteitis, an inflammation of the bone, should be differentiated from osteomyelitis, as direct bacterial invasion of bone in CRS has not yet been demonstrated in studies.
Innate & Adaptive Immune Dysfunction
The innate immune system provides the first line of defense against pathogens through both physical barriers, such as ciliated mucosa, and the expression of several antimicrobial molecules, including S100 and surfactant protein A. The data on these antimicrobial molecules have been somewhat inconsistent. Some studies have not shown consistent changes in these antimicrobial molecules in patients with CRS.[37,38] Other more recent studies have shown more consistent changes, specifically in the S100 proteins.[39] These have direct antimicrobial effects, as well as aid in recruitment of neutrophils and lymphocytes. These proteins are decreased in patients with CRS compared with controls. The dysfunction of the innate immune system remains a strong area of ongoing research to determine its true role in the pathophysiology of CRS.
Dysfunction in the adaptive immune system may also play a role in the development of CRS. Epithelium serves an important role in the adaptive immune system, mediating communication through cell surface molecules that regulate activation of T cells, as well as producing cytokines and chemokines that activate B cells and T cells, and enable their migration. Dysregulation of the interaction between epithelial cells and the adaptive immune system may also play an important role in the development of CRS. Furthermore, free light chains, which are thought to be involved in mast cell-dependent immune responses, have been found to be increased in nasal secretions and mucosal tissue of patients with CRS.[40] This increase is most prominent in CRSwNP. The increased free light chains suggest a possible role in mediating the local immune dysregulation in CRS.
Allergy
Allergy may represent a confounding factor in the development of CRS. Allergy often manifests as swelling of nasal mucous membranes, leading to sinus ostia narrowing and obstruction. Such obstruction can lead to retained mucus, decreased ventilation and infection. Furthermore, positive allergy skin prick tests are highly associated with CRS. Benninger reported 54% of patients with CRS had positive skin prick tests.[41] This is in keeping with multiple other studies, showing rates of positive skin prick tests in 50–84% of patients with CRS undergoing sinus surgery.[42,43] However, other studies point toward no increase in CRS in patients with positive allergic responses. Despite the lack of a clear etiologic role for allergy in CRS, it likely represents a contributing factor that should be addressed in the overall treatment strategy.
Anatomic Factors
Anatomic factors have been theorized to play a role in the development of CRS. These include a pneumatized middle turbinate (concha bullosa), septal deviation and variations in configuration of the uncinate process. Despite the proposed mechanisms of anatomic variability leading to CRS, multiple studies have shown no difference in prevalence of anatomic variations between patients with and without CRS.[44,45] By contrast, a systematic review on the role of septal deviation in CRS concluded that increasing angles of septal deflection were associated with a small, but significant, increasing prevalence of CRS.[46] On the basis of current information, the exact role of anatomic variations in CRS is unclear. It would seem that altered sinus ventilation may result from anatomic variants, but this alone is likely insufficient for the development of CRS.
Diagnosis of CRS
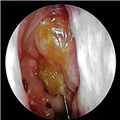
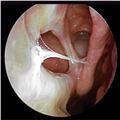
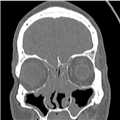
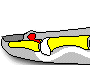
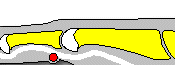
The definition of CRS encompasses symptoms, duration of symptoms and objective findings on examination or imaging. CRS is defined as inflammation of the nose and paranasal sinuses characterized by two or more symptoms, one of which should be either nasal blockage/congestion/obstruction or nasal discharge (rhinorrhea or postnasal drip).[2] Other symptoms include facial pain or pressure, as well as decreased or complete loss of smell. CRS also requires objective evidence of inflammation, identified either on endoscopy or CT. Findings on endoscopy include nasal polyps, mucopurulent drainage or edema/mucosal obstruction, primarily in the middle meatus (Figures 1 & 2). While anterior rhinoscopy may also identify polyps, purulent drainage or polypoid changes, mucosal abnormalities of the middle meatus usually require nasal endoscopy. Furthermore, endoscopy provides detailed information about intranasal anatomy, identification of sinonasal pathology and the ability to obtain endoscopically guided cultures. CT imaging may demonstrate mucosal changes within the ostiomeatal complex or sinuses (Figure 3). Plain sinus films possess a relatively low sensitivity and specificity to identify mucosal changes. By contrast, MRI is too specific and is not currently recommended for the diagnosis of routine CRS.
The objective findings on examination or imaging are requisite in the diagnosis of CRS as studies have shown a poor correlation between symptoms alone and accurate diagnosis. Hwang et al. identified 125 patients undergoing a CT of the sinuses.[47] Of these, 115 met symptom criteria for CRS. The sensitivity of symptom-based criteria detecting a positive scan was 89%. The specificity, however, was only 2%. The poor correlation of CRS with symptoms alone underscores the need for objective confirmation of underlying sinonasal inflammation.
Functional Endoscopic Sinus Surgery
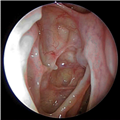
Surgical intervention may be considered in patients with refractory symptoms with objective evidence of disease by endoscopy and/or CT. Surgery does not represent a cure to CRS but rather represents one key intervention in the overall management paradigm of CRS. The goals of surgery are to establish ventilation and facilitate drainage from the paranasal sinuses, to optimize topical delivery of medications directly to the sinonasal mucosa, to reduce inflammatory load by removal of polyps and osteitic bony partitions and to obtain critical diagnostic information from directed cultures and histopathology (Figure 4).
Several studies have analyzed the effectiveness of FESS. A systematic review by Smith et al. examined whether patients with CRS, who have failed medical management, noted improvement in symptoms and/or quality of life after FESS.[76] A total of 45 articles met inclusion criteria for analysis, with the vast majority (n = 42) representing retrospective case series. Two articles represented expert opinion, and one represented a prospective cohort study with a comparison group. All of the articles generally supported the concept that FESS improves symptoms and/or quality of life in adult patients with CRS. A prospective study by Metson and Gliklich reviewed 108 patients undergoing FESS and found significant improvement in symptoms and reduction in medication usage in 82% of patients 1-year postoperatively.[77] Studies have shown consistent improvement, even as long as 10 years after surgery.[78]
A recent prospective, multi-institutional study compared medical versus surgical therapy for CRS.[79] A total of 108 patients were prospectively enrolled into a nonrandomized, multi-institutional cohort. Patients elected continued medical management or FESS and were followed to a primary end point of 6 months. Surgical patients reported significantly greater improvement than medically managed patients, based on validated quality of life questionnaires. Furthermore, surgical patients reported reduced usage of oral antibiotics and steroids and fewer missed days of work/school following FESS.
A second prospective study directly evaluated the effect of FESS on antibiotic utilization.[80] A total of 503 patients were followed for an average of 17.3 months. These patients reported a 57.2% reduction in time on antibiotics following FESS. When evaluating specific subgroups, the antibiotic reduction was identified for patients with CRS both with and without polyps, as well as patients with recurrent ARS.
However, not all published evidence advocates the utility of FESS for CRS. A recent Cochrane review concluded that FESS is not superior to medical treatment in patients with CRS.[81] This included three randomized controlled trials. The first did not compare FESS to medical therapy but rather compared endoscopic middle meatal antrostomy to more traditional inferior meatal antrostomy [Fairley JW, Unpublished Data]. The second randomized patients with CRS into a medical or surgical therapy group.[82] However, this was only after failure of alkaline nasal irrigations and topical corticosteroids, which does not represent maximal medical therapy. In a recent study of ARS members, a majority of respondents reported use of antibiotics and topical nasal steroids 'almost always' representing greater than 90% of the time, with a mean antibiotic length of 3.1–4 weeks.[83] While there is no consensus on the definition of maximal medical therapy, other essential medications in the treatment schema should include nasal saline irrigations and oral corticosteroids, which were not included in the study referenced in the Cochrane review. The third study in the Cochrane review suffered from similar limitations.[84]Patients with chronic maxillary sinusitis were randomized to receive either maxillary sinus irrigation in the office or sinus irrigation followed by FESS within 3 days after enrollment. Each group received 10 days of lorcarbef upon enrollment into the study. Again, FESS patients were not selected based on failure of maximal medical therapy. It is generally accepted that FESS should occur only after medical therapy has failed, making a comparison between medical therapy and FESS somewhat problematic. Thus, the available Cochrane review fails to capture the overwhelming data attesting to the efficacy of FESS for CRS.
Balloon Dilatation
Balloon catheter technology has been presented as a potentially less invasive alternative for patients undergoing sinus surgery. An adaptation of cardiac like devices, the technology cleared the US FDA in 2005. Balloon dilatation employs a noncompliant balloon with the ability to displace bone and tissue to enlarge the sinus ostia. Several studies have sought to clarify the role of balloon dilation. The CLEAR study, published in 2007, is a prospective, multicenter trial of 109 patients with CRSsNP, unresponsive to medical management, undergoing balloon dilatation with or without concurrent ethmoidectomy.[85]Follow-up evaluations were performed at 1, 12 and 24 weeks after surgery. The authors report ostial patency rates of 80.5%, as well as consistent improvement in quality-of-life measures over baseline. However, 52% of these patients underwent FESS as well as balloon sinuplasty, leading to a 'hybrid' group of patients. For those patient undergoing balloon only procedures, their burden of disease was limited, with a Lund-McKay score of 6.1. This radiologic score seeks to quantify disease burden on CT and should be compared with an incidental Lund-McKay score of 4.26 in the general population.[86]Also, the study does not define explicitly the medical or surgical therapy utilized in these patients. Finally, the measurement of ostial patency was not blinded, leading to a likely optimistic view on actual patency. This uncontrolled, observational study does not provide adequate data to determine the role for balloon dilatation.
A balloon registry in 2008 reported on 1036 patients across 27 practices.[87] This reported a 95.2% improvement rate in symptoms, with 3.8% unchanged and 1% worse. Eight complications were reported, including two cerebrospinal fluid leaks and six episodes of minor epistaxis. While this study seems to demonstrate balloon dilatation to be relatively safe, one is unable to draw any definitive conclusions regarding patient improvement. The disease burden and surgery indications were not defined, and no validated symptom measurement tools were utilized.
Balloon catheter technology likely has a role in the management of CRS, but that role has not yet been fully defined. Further controlled studies comparing balloon dilatation to FESS are needed to better understand its applications.
Expert commentary
CRS is a complex disease process without a singular unifying pathophysiology or uniformly accepted treatment strategy. It likely represents a multifactorial disease entity with contributions from various etiologies such as allergy, microbes, biofilms, Staphylococcal superantigen, osteitis and derangements in innate and adaptive immunity. A comprehensive treatment paradigm should entail medical therapy to control inflammation and infection, and targeted surgery, when indicated in medically recalcitrant cases. As a chronic disease model, the treatment should evolve with close partnership between patient and physician to achieve clinical improvements in CRS symptoms and physical and psychosocial wellbeing. Ongoing research in pathophysiologic mechanisms and treatment schemes are absolute imperatives to continue to enhance patient care
Five-year view
CRS continues to be a disease process predisposed for innovation. The next 5 years will likely provide further basic science research into the etiology of CRS on a molecular level. With enhanced understanding of the etiologic mechanisms of CRS, such increased knowledge will lead to more directed therapies to better control the inflammatory process. In addition, the database on the levels of published evidence with respect to the various treatment modalities will be further refined. This would involve more randomized, placebo-controlled trials with sufficient sample size to facilitate clinically relevant conclusions.